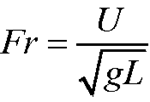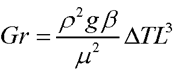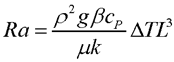Aerospace Micro-Lesson #72
In This Section
Fire in Space
Just about everybody has seen a fire burn, from a candle flame to a small campfire and perhaps something larger. The fuel burns with the oxygen supplied by the air, creating heat; the heated air rises, making room for fresh air to come in and supply more oxygen to continue the fire.
But what happens in space? In the vacuum of space there is no air, so fire as we know it is impossible. But on board a manned spaceship, things can burn using some of the air that the crew is supposed to breathe. But what happens next? This lesson answers that question.
Next Generation Science Standards (NGSS):
- Discipline: Physical Science
- Crosscutting Concept: Scale, Proportion, and Quantity
- Science & Engineering Practice: Constructing Explanations and Designing Solutions
GRADES K–2
NGSS: Motion and Stability: Forces and Interactions: Plan and conduct an investigation to compare the effects of different strengths or different directions of pushes and pulls on the motion of an object.
Fire happens when oxygen, which is a part of the air around us, comes into contact with something that can burn, like wood or paper or wax, and the temperature is high enough—things are hot enough—that they can burn. The burning releases more heat, which makes the things even hotter, and the fire continues. For there to be a fire, you need all three things: the oxygen, the thing that can burn (called “fuel”), and the high temperature.
If you light a candle, it will (usually) burn with a tall, thin flame rising up from the wick. If you hold your hand above the flame—don’t hold it there for too long or it might burn you!—you can feel the hot air rising from the flame. Hot air is less dense than cooler air—there is less of it in any given volume (or you could say that the same amount of it takes up more space). The Earth’s gravity pulling down on the air causes the hot air to “float” in the cooler air around it, much as a helium balloon floats up into the air. As the hot air floats upward from the flame, cooler air comes in from the sides and below to replace it. This cooler air brings more oxygen to feed the flame and the burning continues.
What happens if you do not have the Earth’s gravity pulling down on everything? The hot air does not rise; instead it remains where the flame is and gets in the way of the fresh air coming in. The result is a flame that does not look anything like a normal flame. Instead of being yellow, it is blue; instead of being elongated, it is circular. It is very different and the behavior of fires on the Earth’s surface does not give us a guide to fires in space.
GRADES 3–5
NGSS: Matter and Its Interactions: Make observations and measurements to identify materials based on their properties.
As explained in the K-2 lesson, a fire needs three things in order to burn: oxygen (or, more generally, an “oxidizer”), fuel, and heat. If you take any one of these things away, there can be no fire as we use the term.
If you look at a picture of the Sun taken by one of the spacecraft that study it, though, you will see things on it that look a lot like flames. These things, called “ solar prominences,” are filaments of gas from the surface of the Sun. They can be hundreds of thousands of miles long, more than ten times the size of the Earth! Like flames of fire, they are made of gas; like flames, they are so hot that they glow brightly. But unlike flames of fire, they are not created by burning a fuel in an atmosphere. Instead, the Sun is powered by a process called “ nuclear fusion.” This is very different from a fuel burning in the presence of oxygen.
What solar prominences and ordinary flames have in common is that they are made up of a state of matter called “plasma.” (Depending on how much biology the students know, you may want to note that this is a completely different thing from the blood plasma in our bodies.) In school we learn about the three states of matter—solid, liquid, and gas—and in our everyday lives, that is all there are. But when a gas gets very hot, it starts to glow and the molecules that make it up start to break apart. Scientists often think of this hot, glowing gas as a separate state of matter from ordinary gases like air and water vapor. Plasmas behave differently from ordinary gases; besides being very hot, they also conduct electricity easily and are influenced by electrical and magnetic fields. You do not hear much about them because they are not anything that you would touch or handle.
A true fire, then, resulting from fuel, oxidizer, and heat coming together, is very rare—or may never happen at all by itself—in outer space. Of fuel there is plenty: almost three-quarters of the mass of the universe (excluding dark matter) is hydrogen, which burns readily in the presence of oxygen and heat. Oxygen, although it is the third-most-abundant element in the universe, makes up only about one percent of the universe’s mass. The missing part is heat: virtually all of the universe is either extremely cold, out between the stars and galaxies, or, inside of stars, hotter than any fire could make it.
GRADES 6–8
NGSS: Matter and Its Interactions: Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
NASA has created a video that shows the burning of a droplet of liquid fuel in microgravity. It shows a virtually invisible spherical blue flame with isolated pieces of soot burning much hotter and occasional jellyfish-like pulsations from the surface of the burning droplet. Years earlier, some Soviet cosmonauts also created a fire in space, lighting a match and a candle and filming what they saw. The match and candle flame are yellow, not blue, and while they were somewhat rounder than an Earth-based candle flame and there was no specific “up” direction, the flames look almost as if they could have been created from a fire on Earth. Why the difference?
You can figure out the reason by thinking carefully about what shapes a flame on Earth. As described in the earlier lessons, the typical candle flame gets its shape from the flow of the hot air containing the burned products upward away from the fire because of the buoyancy of the hot air relative to the colder air around it. In zero gravity, there is no buoyancy. But buoyancy is not the only way to make the hot air flow away from the flame. Even on the Earth’s surface, a light wind will move the hot air and burned products away from the flame. (A flame in still air is a lot longer than a flame in a wind because the wind causes the hot air to mix more efficiently, cooling it down to a temperature that is low enough that it does not glow anymore.) Look again at the video of the Soviet cosmonaut. When you see the first flame on the match, the cosmonaut is moving it around relative to the air around it, bringing it into contact with fresh air and allowing the fire to burn more efficiently. (You can see the flame brighten as it comes into the fresher air at the end of each “wave” in its motion.) Later in the video, you can see the cosmonaut blowing gently on the candle flame, moving the burned products away and replenishing its supply of fresh air. The NASA video, in contrast, shows a stationary droplet burning in still air. With no other means of bringing fresh air into contact with the burning droplet, the flame is blue and the droplet burns relatively slowly. (Notice how, around 1:45 and again at 2:05 in the NASA video, when the droplet is first ignited it burns brightly. This is because it is using up the oxygen in the air right next to it. After a few seconds, all the nearby oxygen is used up and the fire must wait for oxygen to diffuse over to it from farther away. The rate of burning goes down and the flame gets much dimmer.)
GRADES 9–12
NGSS: Matter and Its Interactions: Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
Most fires in space that have been deliberately set—and thankfully there have been precious few accidental fires in space—have, for the most part, been rather small. Fires use up oxygen, after all, and oxygen is precious in space. Three exceptions to this are NASA’s Saffire experiments, conducted in 2016 and 2017 aboard Cygnus supply spacecraft after they had finished delivering their cargoes to the Space Station. After the Cygnus craft had undocked from the Space Station, the experiment involved igniting a cloth of some square feet in area and photographing how the flames spread. NASA’s Saffire experiment page has links to web pages with pictures and videos of the experiment.
While the reason should be obvious for waiting to perform the experiment until the Cygnus spacecraft had separated from the Space Station and was on its way back down to Earth, destined to burn up in the Earth’s atmosphere, it may still be worthwhile to ask the students for suggested reasons.
One reason for wanting to experiment with larger fires in space is that fires of different sizes behave differently. The flame from a candle (without a significant wind) is smooth and laminar; a campfire is chaotic and turbulent; a burning building is literally an inferno. The airflow in a fire is governed by several different dimensionless constants which describe the relative importance of different phenomena:
- Prandtl Number –

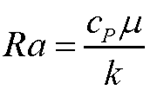 – the ratio of the importance of viscosity (momentum diffusion) to thermal diffusion
– the ratio of the importance of viscosity (momentum diffusion) to thermal diffusion
In these equations, “ρ” is the density of the air, “U” is a typical flow speed, “L” is a typical length scale of the flow,"μ” is the viscosity of the air, “g” is the gravitational (or similar) acceleration, “β” is the coefficient of thermal expansion of the air, “ΔΤ” is the difference in temperature between the regular air and the flame, “cP” is the specific heat of the air at constant pressure (the heat the air absorbs to raise its temperature by a single degree, keeping the pressure constant), and “k” is the thermal diffusion coefficient of the air.
These numbers are not all independent of each other and there are other numbers as well. Scientists studying fires in space want to extend the ranges over which they study fires so that they do not miss important phenomena that might happen at one scale but not another.
Sixty Years Ago in the Space Race:
October 15, 1958: The United States’ X-15 hypersonic experimental airplane was rolled out and shown to the public for the first time.